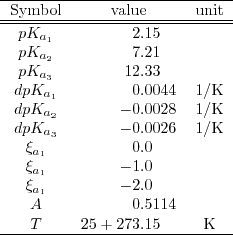
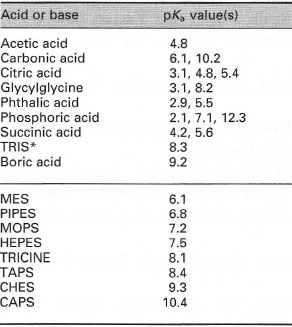
![PDF] Buffer solutions in drug formulation and processing: How pKa values depend on temperature, pressure and ionic strength | Semantic Scholar PDF] Buffer solutions in drug formulation and processing: How pKa values depend on temperature, pressure and ionic strength | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/879d3eae13956041f4cc1b8ee223109275087b7e/3-Table1-1.png)
PDF] Buffer solutions in drug formulation and processing: How pKa values depend on temperature, pressure and ionic strength | Semantic Scholar
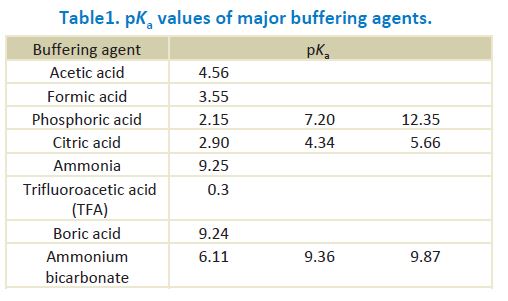
![PDF] The Influence of Ionic Strength on Apparent and Thermodynamic Parameters ( Ka , pKa ' s ) for HF and Phosphate Buffer Capacities | Semantic Scholar PDF] The Influence of Ionic Strength on Apparent and Thermodynamic Parameters ( Ka , pKa ' s ) for HF and Phosphate Buffer Capacities | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c0a6140f88db06398f56b28ce5fd6ac92cd82d39/5-Table3-1.png)
PDF] The Influence of Ionic Strength on Apparent and Thermodynamic Parameters ( Ka , pKa ' s ) for HF and Phosphate Buffer Capacities | Semantic Scholar
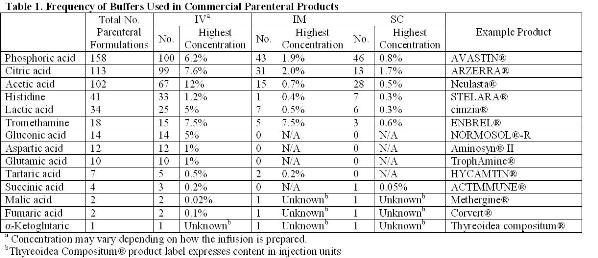
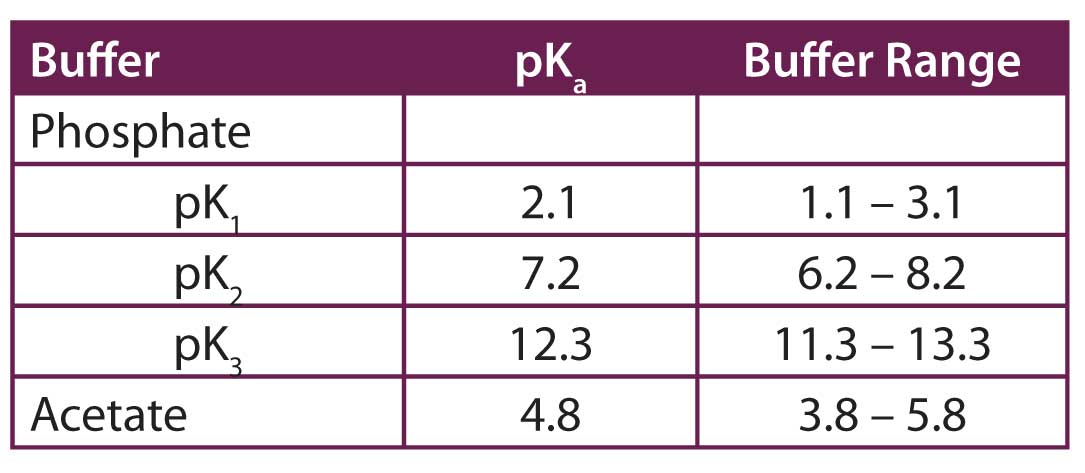
Breaking old habits: Moving away from commonly used buffers in pharmaceuticals - European Pharmaceutical Review
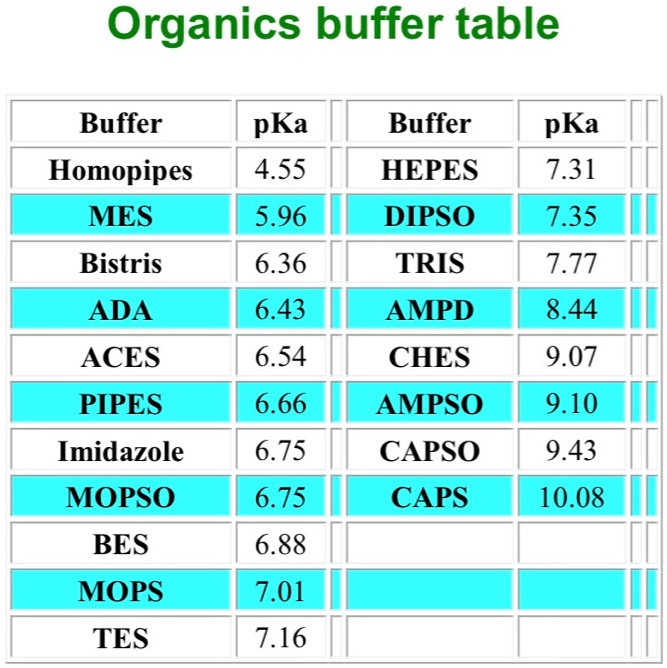
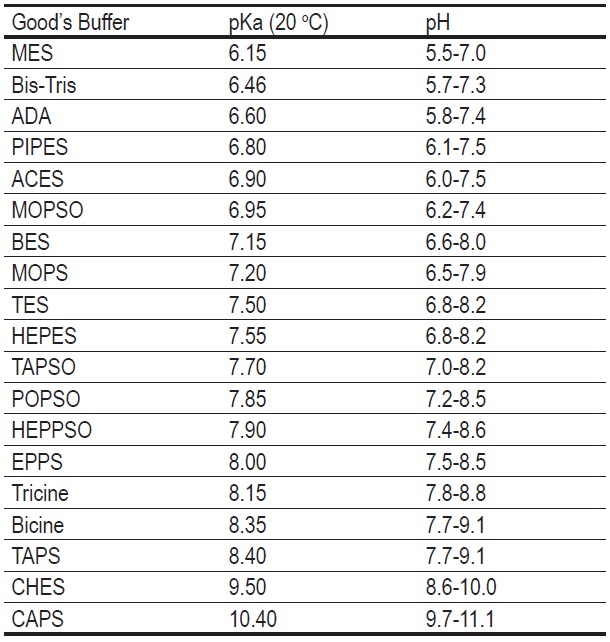
SOLVED: Organics buffer table Buffer Buffer Homopipes MES pKa 4.55 5.96 HEPES pKa 7.31 7.35 DIPSO Bistris pKa 6.36 TRIS AMPD pKa 7.77 ADA pKa 6.43 8.44 ACES PIPES pKa 6.54 CHES

Solution pKa values for a range of polymers at various polymer and salt... | Download Scientific Diagram

equilibrium - Why do buffers need to be composed of equal amounts of the acid and salt? - Chemistry Stack Exchange

OneClass: A buffer solution is prepared by mixing 43.1 mL of 0.0478 M sodium dihydrogen citrate with ...
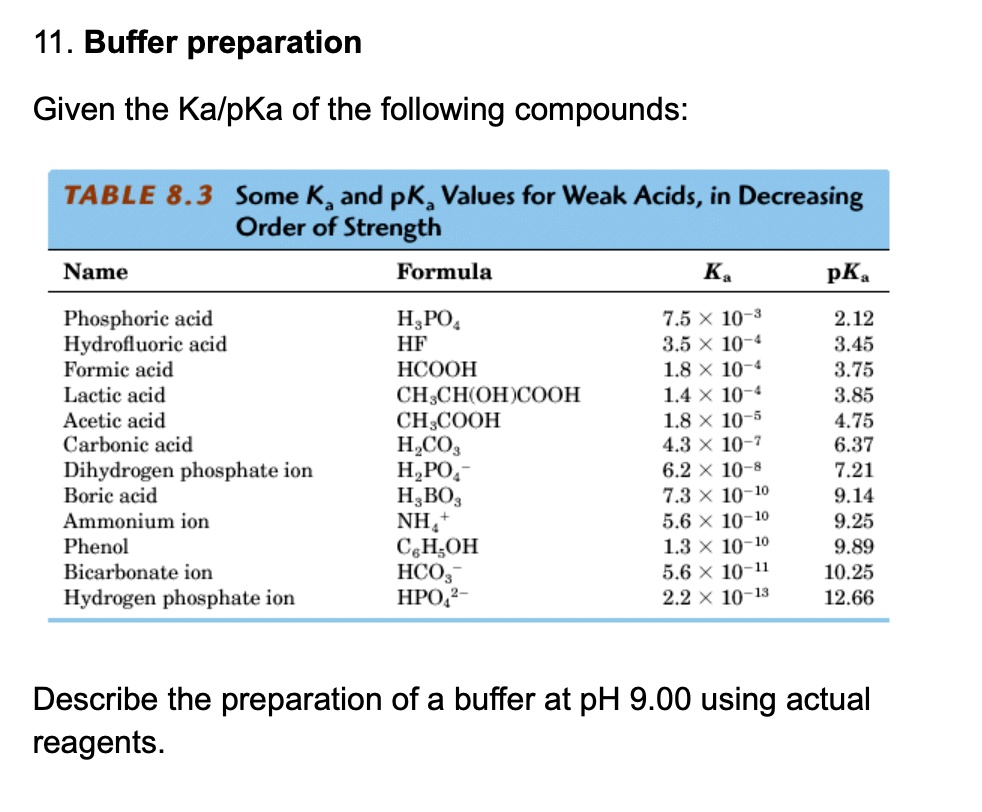
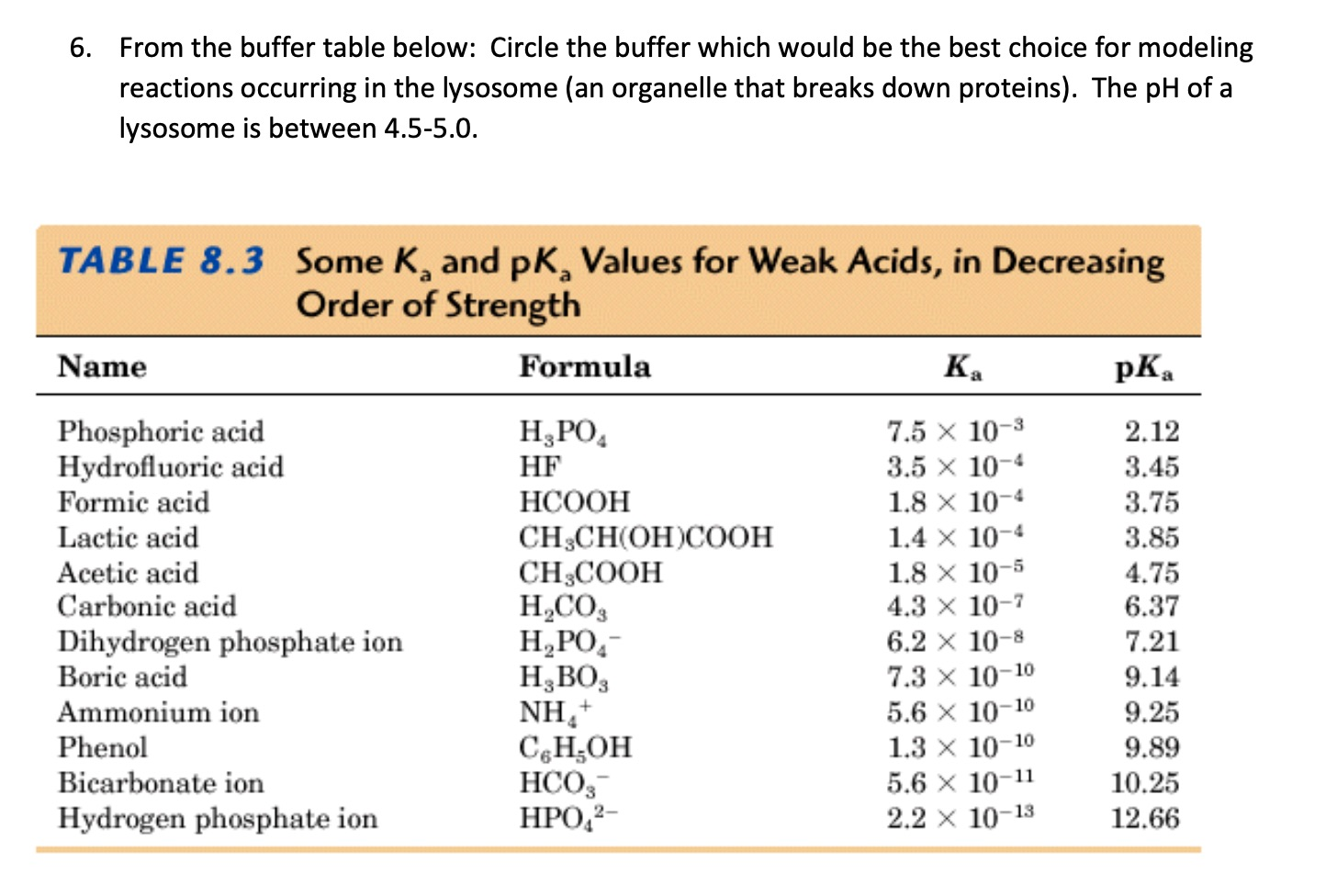
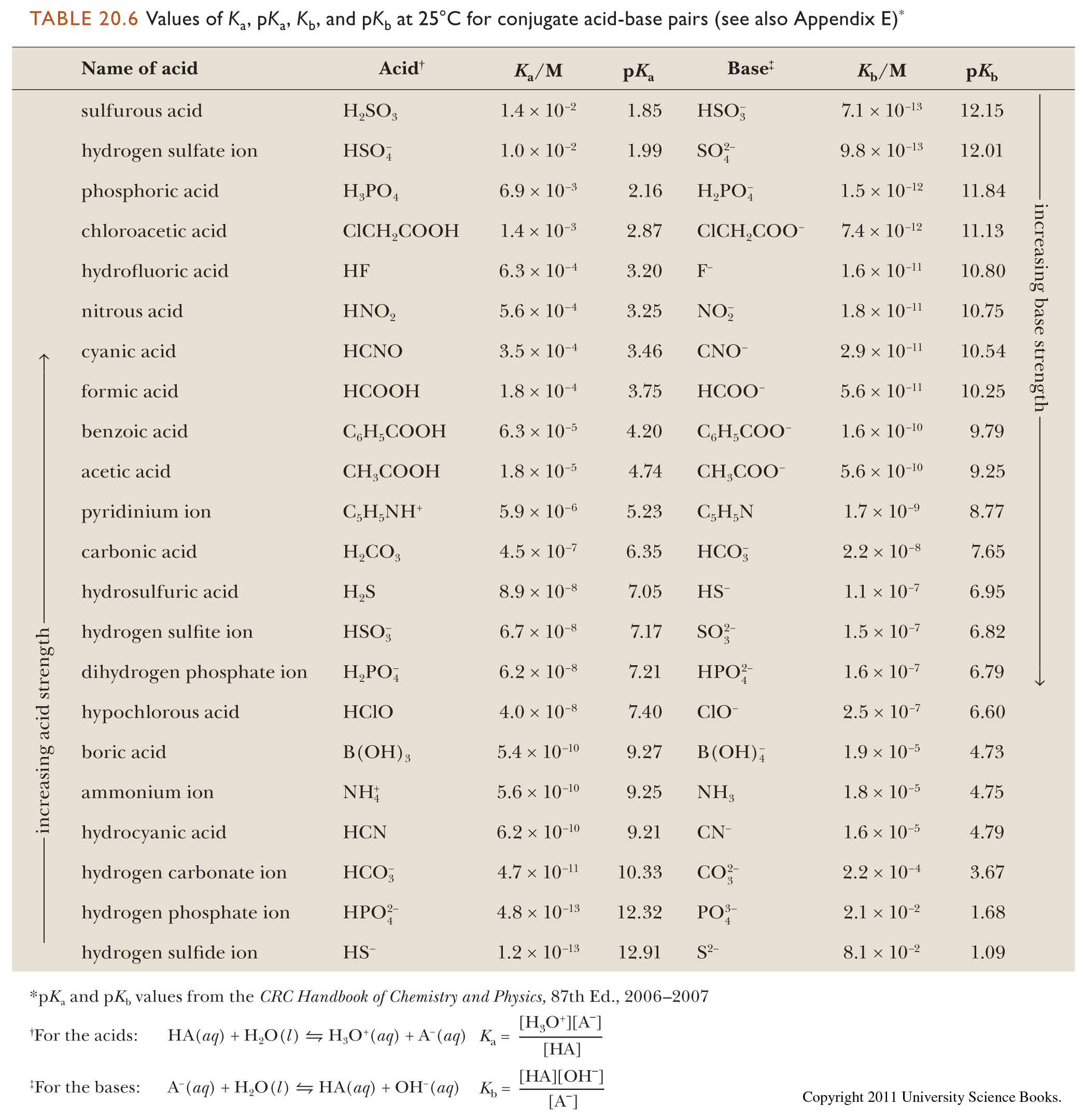
SOLVED: 11. Buffer preparation Given the KalpKa of the following compounds: TABLE 8.3 Some K; and pK; Values for Weak Acids, in Decreasing Order of Strength Name Formula K pK. Phosphorie acid
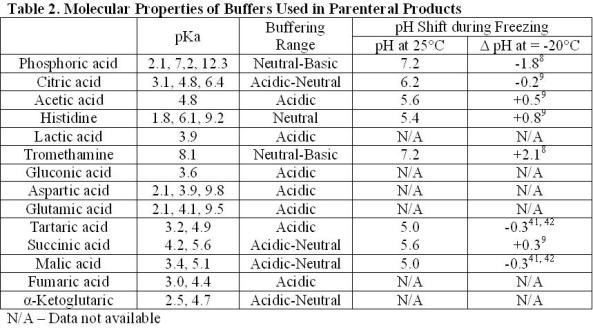
Breaking old habits: Moving away from commonly used buffers in pharmaceuticals - European Pharmaceutical Review

Chemical composition of some buffer solutions covering the pH range of... | Download Scientific Diagram

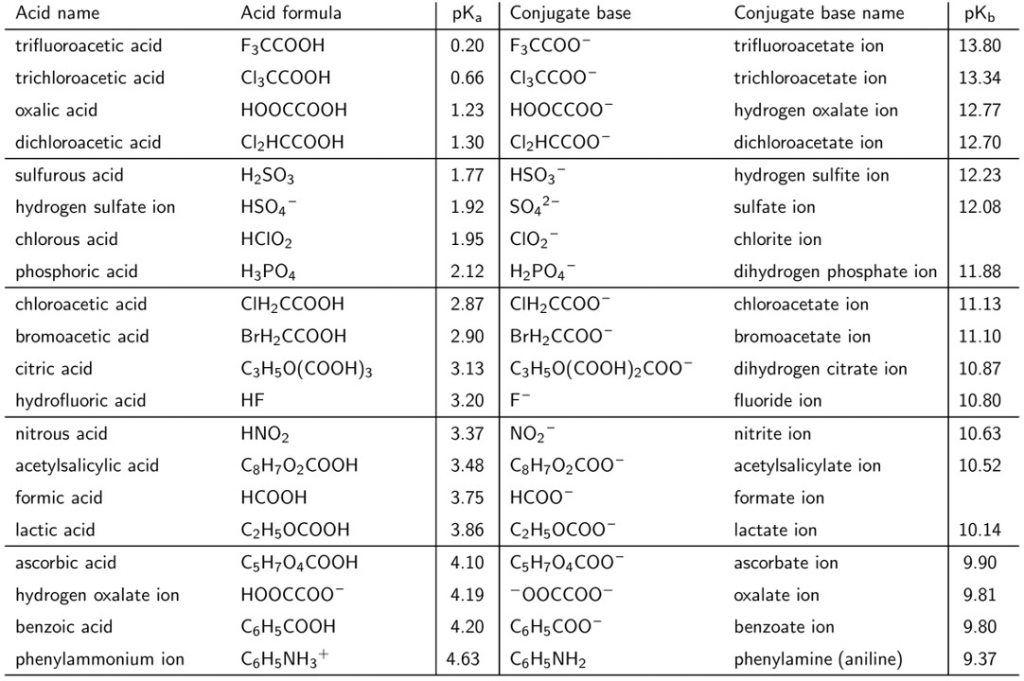
A Reliable and Efficient First Principles-Based Method for Predicting pKa Values. 2. Organic Acids | The Journal of Physical Chemistry A