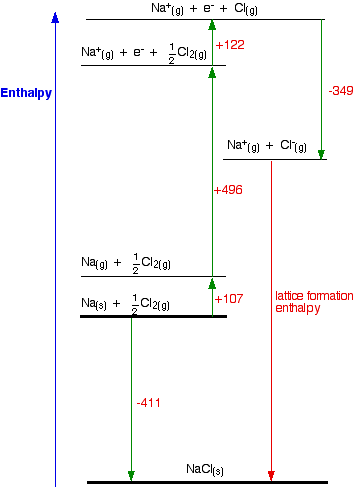
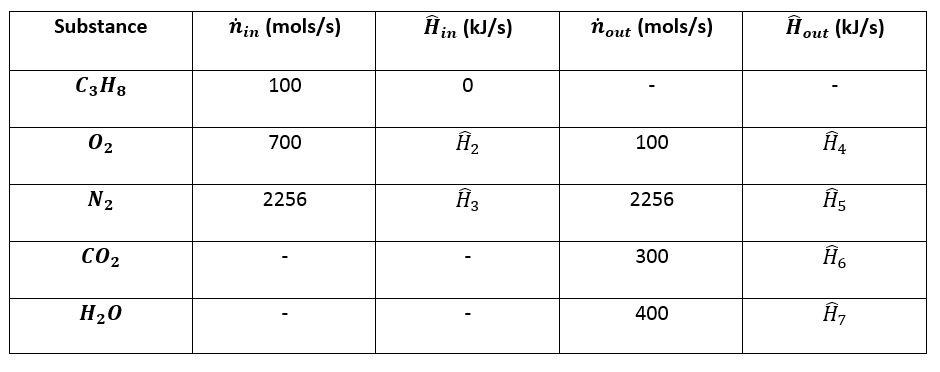
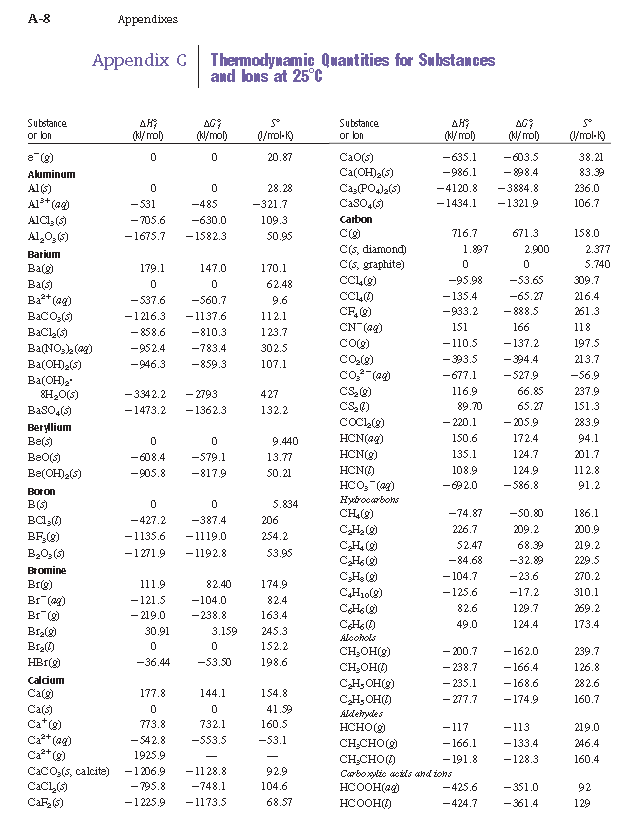
Table 6 from Structure and heats of formation of iodine fluorides and the respective closed-shell ions from CCSD(T) electronic structure calculations and reliable prediction of the steric activity of the free-valence electron

Table 3 from Group additivity values for enthalpies of formation (298 K), entropies (298 K), and molar heat capacities (300 K < T < 1500 K) of gaseous fluorocarbons | Semantic Scholar
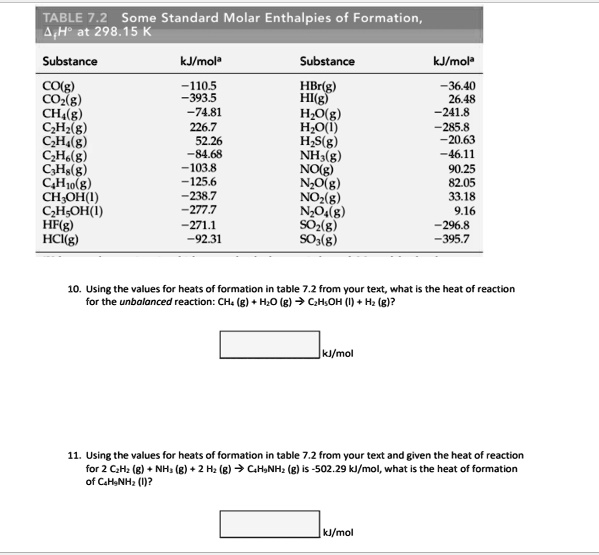
SOLVED: TABLE 7.2 Some Standard Molar Enthalpies of Formation; Heat at 298.15 K Substance kJ/mol Substance kJ/mol CO(g) CO2(g) CH4(g) C2H6(g) C3H8(g) C4H10(g) C6H6(g) C6H12(g) C6H14(g) C6H6OH(l) C2H5OH(l) -105 -393.5 -74.81 226.7

2009, Prentice-Hall, Inc. Enthalpies of Formation An enthalpy of formation, H f, is defined as the enthalpy change for the reaction in which a compound. - ppt download
![Appendix D. Heats of Formation and Combustion - Basic Principles and Calculations in Chemical Engineering [Book] Appendix D. Heats of Formation and Combustion - Basic Principles and Calculations in Chemical Engineering [Book]](https://www.oreilly.com/api/v2/epubs/9780132885478/files/graphics/appd-tab-d1a.jpg)
Appendix D. Heats of Formation and Combustion - Basic Principles and Calculations in Chemical Engineering [Book]

physical chemistry - How do you calculate the heat of a reaction given a table of heat of formation values? - Chemistry Stack Exchange

The question asks what is Using the provided table and the equation below, determine the heat of formation - brainly.com

Table 5 from The Heats of Chemical Reactions: the Van't-Hoff Equation and Calorimetry | Semantic Scholar
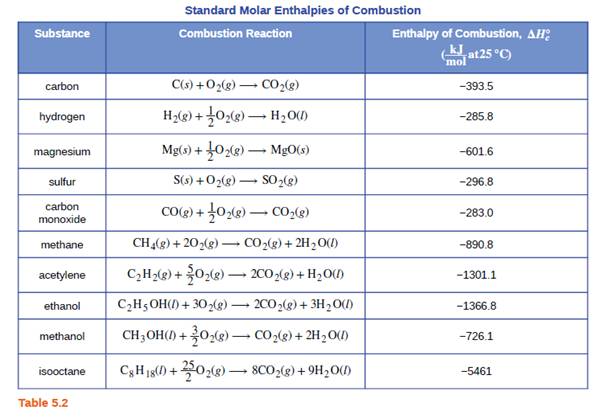
Which of the enthalpies of combustion in Table 5.2 the table are also standard enthalpies of formation? | bartleby
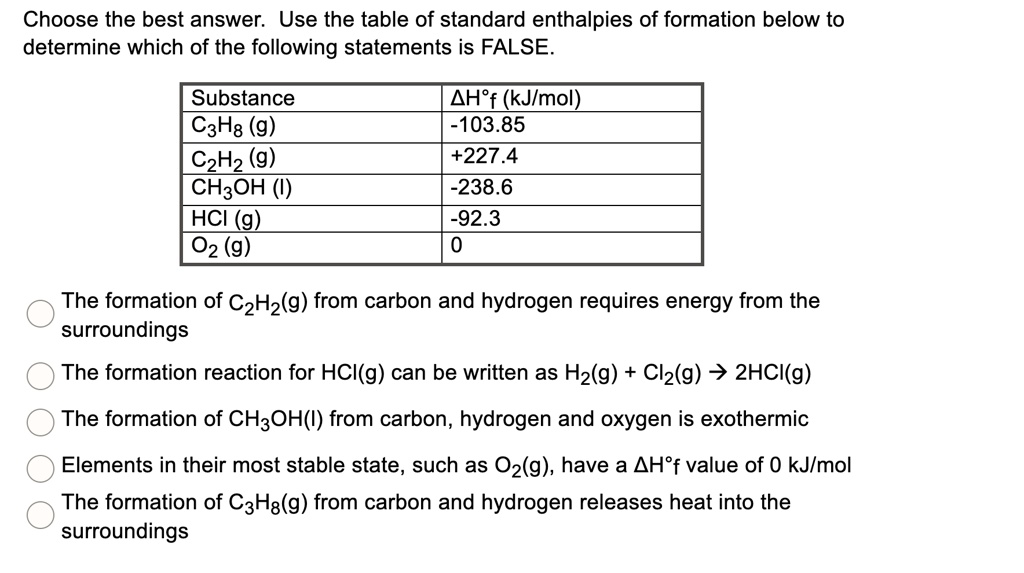
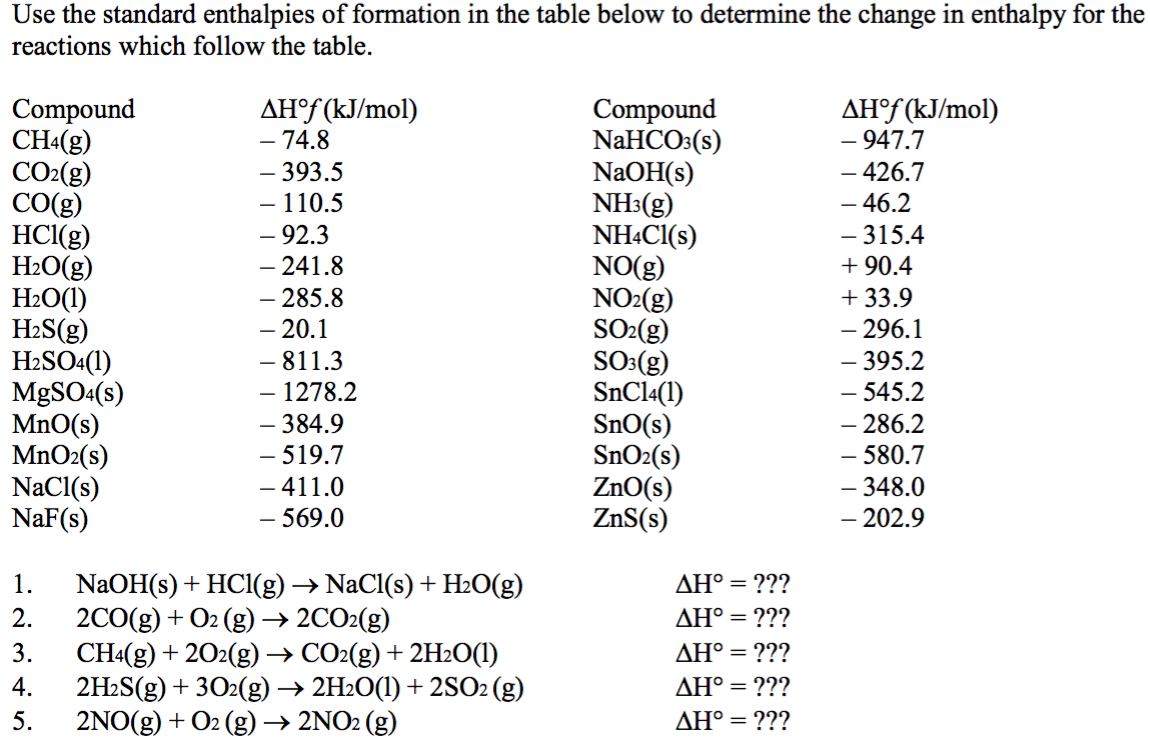
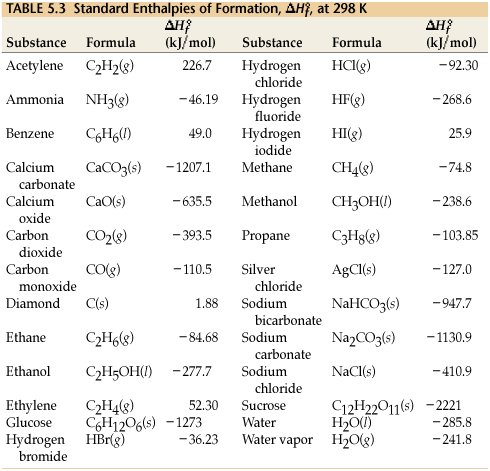
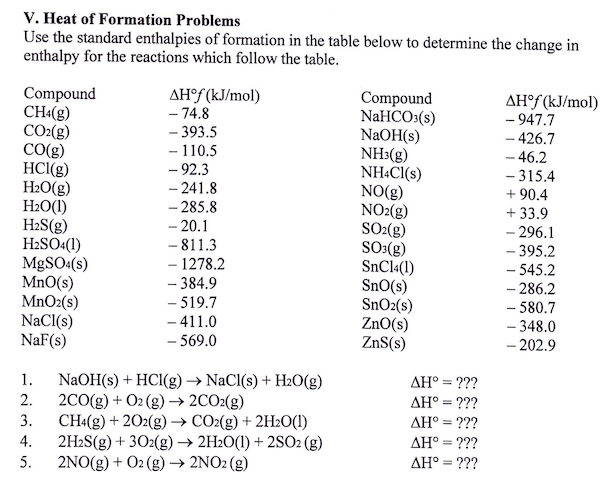




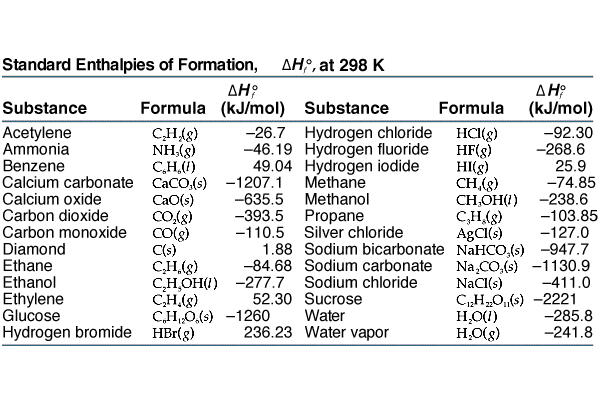
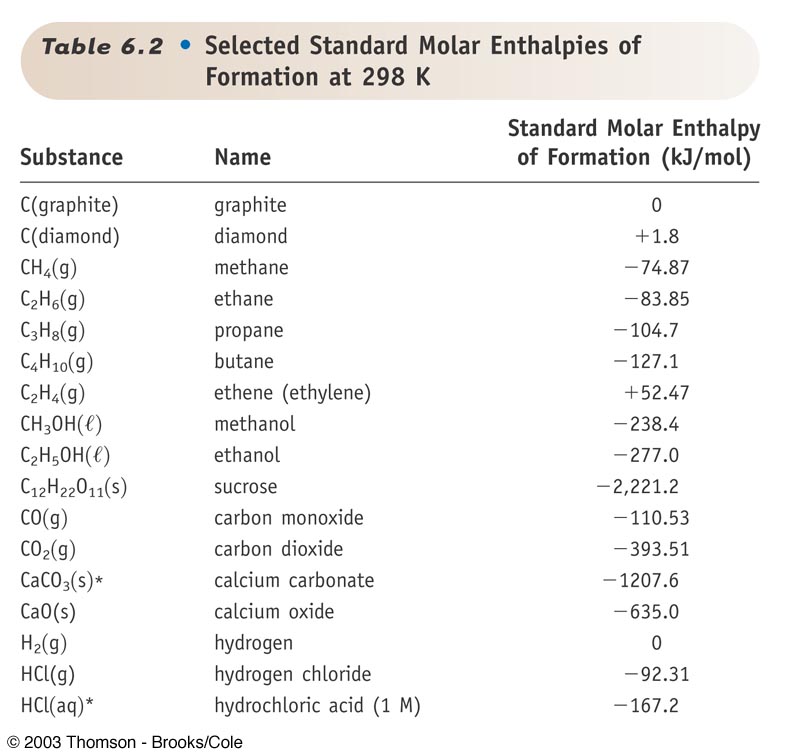
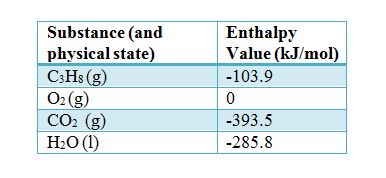
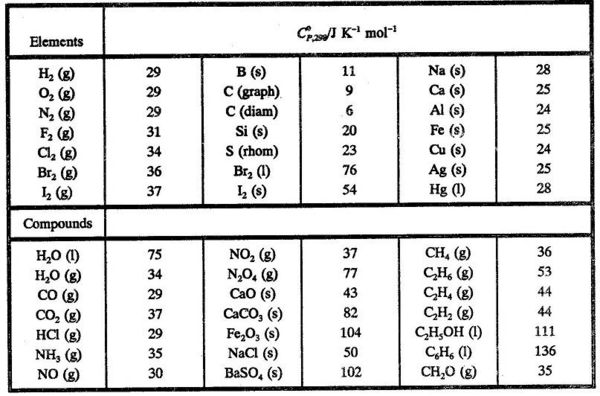
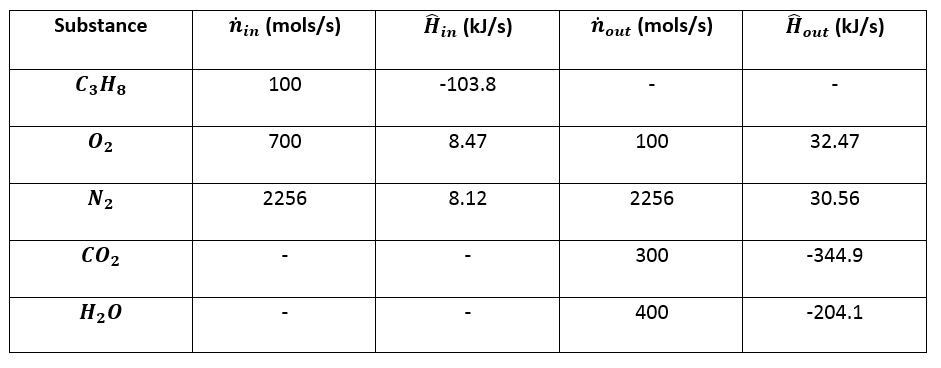

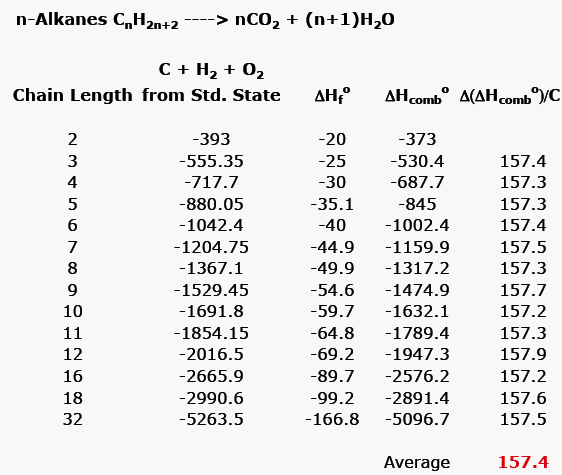
:max_bytes(150000):strip_icc()/GettyImages-154953454-6806780a2f0f4ec99daf580619b5aeef.jpg)